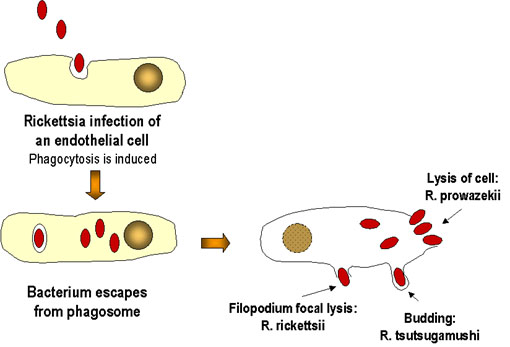
|
Rickettsia conorii is the infectious agent which
causes fievre boutonneuse (Mediterranean fever) in man. This disease is an
eruptive fever transmitted by the bite of the brown dog tick.
Rickettsia conorii is an obligate intracellular parasite which
multiplies in the cytoplasm of eukaryotic cells; its genome has a size of 1.2
Mb. These bacteria have been closely associated with arthropods for several
hundred million years. Rickettsia conorii is a similar but distinct
species from Rickettsia prowazeki, which has already been sequenced;
it belongs to the alpha branch of proteobacteria, as defined by comparison of
16S RNA sequences.
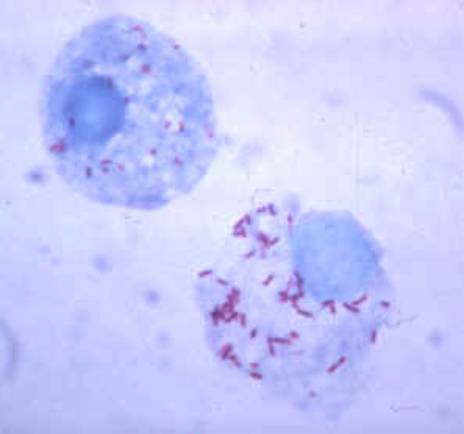
Classification
Higher order taxa:
Bacteria; Proteobacteria; Alphaproteobacteria; Rickettsiales; Rickettsiaceae;
Rickettsieae
Species:
Orientia tsutsugamushi; spotted fever group: Candidatus
Rickettsia principis; Israeli tick typhus rickettsia; Rickettsia
aeschimannii; Rickettsia africae; Rickettsia akari; Rickettsia amblyommii;
Rickettsia andeana; Rickettsia australis; Rickettsia conorii; Rickettsia
cooleyi; Rickettsia felis; Rickettsia heilongjiangensis; Rickettsia
heilongjiangii; Rickettsia helvtica; Rickettsia honei; Rickettsia hulinensis;
Rickettsia hulinii; Rickettsia japonica; Rickettsia martinet; Rickettsia
massiliae; Rickettsia monacensis; Rickettsia montanensis; Rickettsia moreli;
Rickettsia parkeri; Rickettsia peacockii; Rickettsia rhipicephali; Rickettsia
rickettsii; Rickettsia sibirica subgroup; Rickettsia slovaca; Rickettsia
sp.; Typhus group: Rickettsia canadensis; Rickettsia prowazekii;
Rickettsia typhi; unclassified Rickettsia: Candidatus
Rickettsia tarasevichiae; Rickettsia bellii; Rickettsia publicis; Rickettsia
sp.
Description and Significance
Rickettsia bacteria are well known
pathogens. Rickettsia conorii causes Mediterranean spotted fever in
humans and is contracted by contact with infected brown dog ticks. Other
Rickettsia include Rickettsia prowazekii, which causes typhus, R.
rickettsii, which causes Rocky Mountain spotted fever, and Rickettsia
akari, which causes rickettsialpox. In addition to this, the genome of Rickettsia
prowazekii is similar to mitochondrial genomes; phylogenetically, R.
prowazekii is more closely related to the mitochondria than any other
microbe known thus far.
Genome Structure
The genome of Rickettsia prowazekii is 1,111,523 base pairs in length
and contains 834 protein-coding genes. It contains no genes for anaerobic
glycolysis as well as genes involved in the biosynthesis and regulation of
biosynthesis of amino acids and nucleosides in free-living bacteria similar
to mitochondrial genomes. Unlike the mitochondrial genome, however, the
genome of R. prowazekii contains a complete set of genes encoding for
the tricarboxylic acid cycle and the respiratory-chain complex. Still, the
genomes of Rickettsia as well as the mitochondria are small, highly
derived, "products of several types of reductive evolution"
(Andersson et al. 1998).
Cell Structure and Metabolism
|
Rickettsia bacteria are obligate intracellular pathogens that are dependent on
entry, growth, and replication within the cytoplasm of a eukaryotic host
cell. The host cell then lysis and releases the rickettsial progeny to
initiate a new infection cycle. The infection generally doesn't result in
complete shutdown of the host machinery. Apparently, "vigorous host
responses" generally clear the rickettsial pathogens (Radulovic et
al. 2001). Conversely, the host's immune responses can also lead to the
persistence of a subclinical infection even years past primary infection
and/or antibiotic treatment. One theory on how rickettsiae survives in host
cells has to do with the "suppression of the antimicrobial activities
of the eukaryotic target cells, specifically monocytes/macrophages"
(Radulovic et al. 2001).
|
|
Ecology
Rickettsia bacteria are commonly carried by anthropods like ticks, mites,
lice, or fleas. Another way for animals and humans to contract the bacteria
is through wild rodents that have been infected with a Rickettsia
bacteria by these louse. Different forms of Rickettsia and the
diseases that they cause can be found all over the world. While some
diseases themselves are worldwide, others, such as Oriental spotted fever
caused by R. japonica in Japan, are localized to one general place
or area.
|
 
Male and female brown dog ticks (Rhipicephalus sanquineus)
that are known to carry Rickettsia. From the Texas Department of Health
|
Pathology
|
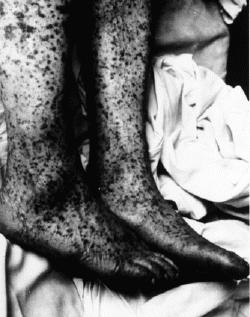
Spotted rash on the legs of patient late in the development of Rocky
Mountain spotted fever. From the CDC
|

Picture of person protecting himself from tick exposure by wearing
light-colored clothing, which allows easy visualization of the ticks, and
tucked pant legs, which deters ticks from crawling inside his pant legs.
From the CDC
|
R. prowazekii is most known for being the agent of epidemic,
louse-borne typhus in humans. It has infected approximately 20-30 million
humans during World War I and killed another few million after World War II
(Andersson et al. 1998). Typhus 'ranks as one of the main epidemic
diseases of human history, a truly apocalyptic pestilence that follows in the
wake of wars, famine, and other human misfortune" (Gray 1998). Rocky
Mountain spotted fever, which is caused by infection with R. rickettsii,
is the most severe rickettsial illness that is tickborne in the US. The
primary ticks that carry it are the American dog tick (Dermacentor
variabilis) and the Rocky Mountain wood tick (Dermacentor andersoni).
Patients infected with R. rickettsii generally have nonspecific
symptoms including fever, nausea, vomiting, muscle pain, lack of appetite,
and severe headache after an incubation period about 5-10 days following an
infected tick bite. Later symptoms include rash, abdominal pain, joint pain,
and diarrhea. Fever, rash, and a previous tick bite are usually the most
common components of clinical diagnosis. Rocky Mountain spotted fever is
treated by a tetracycline antibiotic like doxycycline; once a person has had
the disease, they are thought to have long lasting immunity against re-infection
(CDC).
Below are tables of different groups of Ricketsia along with
the diseases that each species cause and their general geological
distribution. From The University of South Carolina
Spotted Fever Group
|
Organism
|
Disease
|
Distribution
|
|
R. rickettsii
|
Rocky Mountain spotted fever
|
Western hemisphere
|
|
R. akari
|
Rickettsialpox
|
USA, former Soviet Union
|
|
R. conorii
|
Boutonneuse fever
|
Mediterranean countries, Africa,
India, Southwest Asia
|
|
R. sibirica
|
Siberian tick typhus
|
Siberia, Mongolia, nothern
China
|
|
R. australis
|
Australian tick typhus
|
Australia
|
|
R. japonica
|
Oriental spotted fever
|
Japan
|
Typhus Group
|
Organism
|
Disease
|
Distribution
|
|
R. prowazekii
|
Epidemic typhus
Recrudescent typhus
Sporadic typhus
|
South America and Africa
Worldwide
United States
|
|
R. typhi
|
Murine typhus
|
Worldwide
|
Scrub typhus group
|
Organism
|
Disease
|
Distribution
|
|
R. tsutsugamushi
|
Scrub typhus
|
Asia, northern Australia,
Pacific Islands
|
References
General:
- Reinert, Birgit. 2001. "Insights into genome
evolution: The sequence of Rickettsia conorii." Genome News Network
- Andersson, Siv G. E., Alireza
Zomorodipour, Jan O. Andersson, Thomas Sicheritz-Ponten, U. Cecilia M.
Alsmark, Raf M. Podowski, A. Kristina Naslund, Ann-Sofie Eriksson,
Herbert H. Winkler, and Charles G. Kurland. 1998. "The genome sequence of Rickettsia
prowazekii and the origin of mitochondria." Nature, vol. 396.
Macmillan Publishers Ltd. (133-143)
Cell Structure and Metabolism
Pathology:
|